
The application of quantum theory explained a similar splitting effect, known as the so called "anomalous Zeeman effect", which is due to the different quantised spins of the individual charged electrons giving them different magnetic moments which react with the applied magnetic field to produce a wider, finer spectrum of multiple sub-states of equal energy. Known as the "nornal Zeeman effect", it was developed before the quantum effects had been discovered and corresponds to the properties of "zero spin" electrons. The example opposite shows how the atomic absorption spectrum is affected by the application of the magnetic field. The effect was first observed by Zeeman in 1896 and is named after him. When an atom is placed in a strong static magnetic field its spectral lines are split into several components due to the distortion of the shape of the electron orbitals caused by the magnetic field which in turn changes the spacing of the energy levels of the atom. The missing photons will leave corresponding gaps in the spectrum of the white light source which appear as four dark lines on the spectrum with exactly the same characteristic frequencies as in the emission spectrum. In the case of white light passing through a sample of hydrogen vapour with its electrons in the ground state, the energy of the photons in the light beam with frequencies corresponding the hydrogen's energy levels will be absorbed by the hydrogen atoms, causing their electrons to jump to higher quantised energy levels. This describes the effect on a beam of white light passing through a cool (unstimulated) sample of vapour of a particular element. The emission spectrum shown has 4 distinct bright spectral lines or colours with frequencies corresponding to quantum electron jumps from one of four excited states down to the ground state. In the case of the hydrogen atoms, in the example above each individual atom in the sample has five potential quantised energy bands, only one of which is occupied by an electron at any time. This is the light generated by stimulating a source consisting of a sample of single element type. White light consists of a continuous spectrum of frequencies resulting from so called black body radiation which encompasses radiation emitted from multiple different elements and the electron jumps between their multiple energy bands.
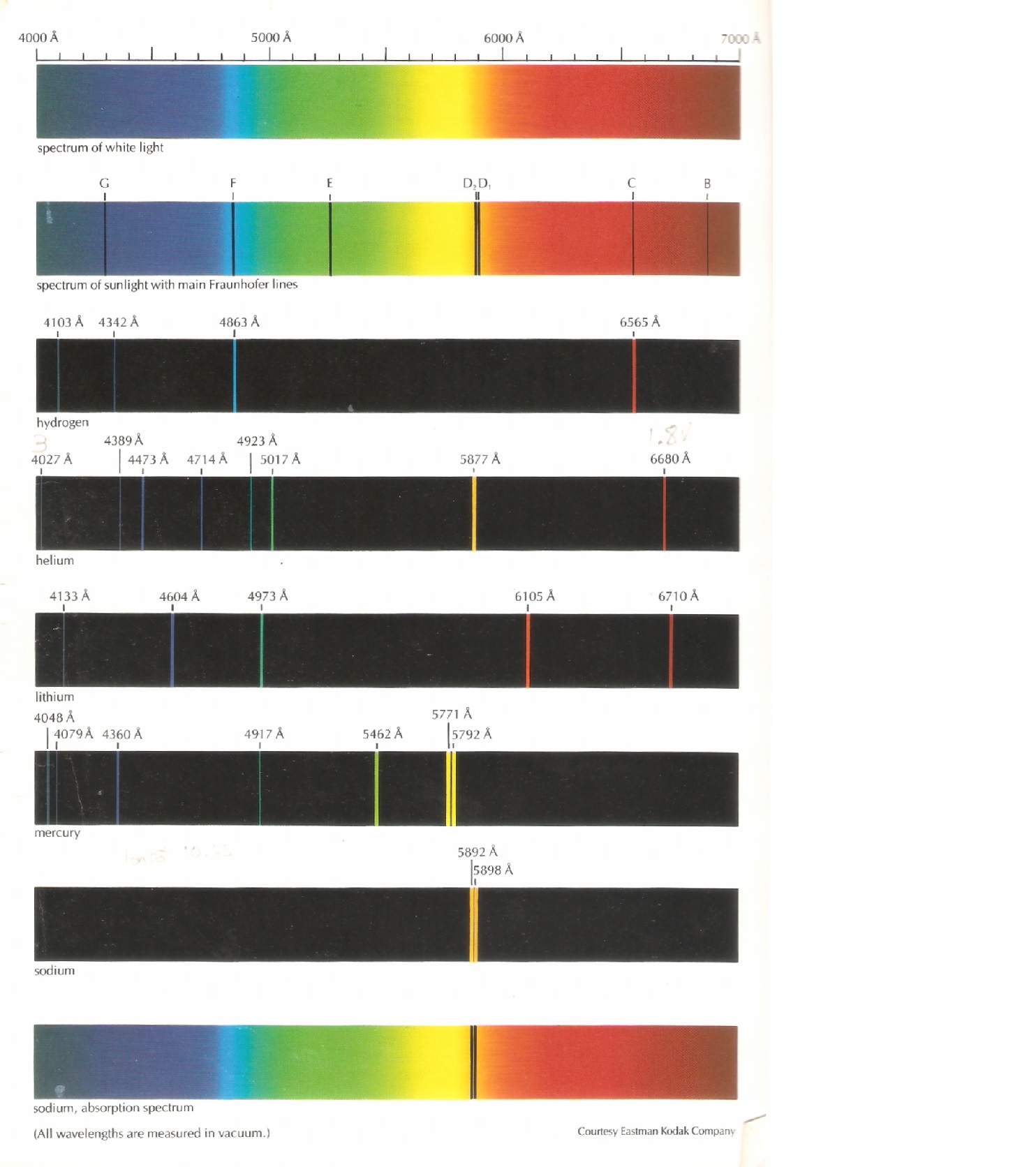
In the hydrogen spectrum opposite, the spectral line with a wavelength of 656 nm (or frequency of 457,000 GHz) corresponds to an energy difference of 4.5 x 10 -5 eV between the electron's ground state and its excited state, known as the fine structure enrgy gap.
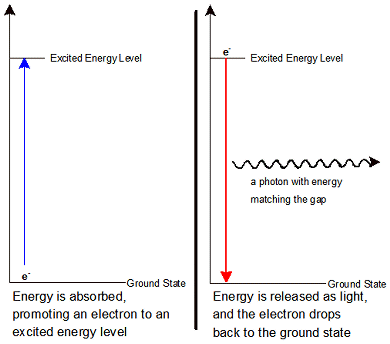
They are the frequencies associated with the energy released or absorbed when an electron jumps from one discrete energy level to another. The spectral lines of light emitted by an atom are known as its characteristic frequencies. The dark lines in the Sun's radiation spectrum were first catalogued by Fraunhofer in 1814 and subsequently investigated by Kirchhoff and Bunsen who provided the initial analysis an explanation of atomic spectral lines. The corresponding wavelength λ is given by λ = c/f where c is the speed of light. The frequency f of the light energy released or absorbed in the quantum jump is given by Planck's Law E= hf where E is the difference in energy levels between the initial orbit and the final orbit and h is Planck's constant. Heat or other stimuli may cause an electron to break out of its energy band and jump over one or more energy bands down to a lower energy orbit releasing a photon with a quantum of energy corresponding to the difference in energy level between the two orbits.Ĭonversely, when photons collide with an atom, their energy may be transferred to and absorbed by the atom's electrons causing them to jump to a higher energy level orbit.
N is also known as the quantum number representing the energy state of the atom.

progressively further away from the nucleus have correspondingly higher energy levels. The shell or orbit n1 closest to the nucleus is known as the ground state and has the lowest energy level. The shells and the electrons in them have characteristic quantised energy levels. Each shell may carry one or more electrons or none at all. Woodbank does not monitor or record these emailsīohr's model of the atom envisaged a nucleus around which electrons circulate in one or more distinct orbits (also called shells or energy bands or more recently orbitals).


 0 kommentar(er)
0 kommentar(er)
